NOW AVAILABLE
Visit the first terpene bar of its kind!
Terpenes for customization of your CBD extracts.
CANNABINOIDSThe first thing to understand is that although most people think of Cannabinoids and CBD as the same thing. This is not the case, CBD which stands for Cannabidiol is just one of over 100 different types of cannabinoids that are contained within the different cannabis plants.
A cannabinoid is a class of different chemical compounds that affect cannabinoid receptors in the body to help with a variety of health benefits to the body. As more research is being done on the Cannabis botanical, more amazing cannabinoids, (molecules, chemical components) are being discovered. It's not just about THC or even CBD anymore. The cannabis plant contains many different cannabinoids, we have seen over 80+ different and it seems there are many more. This is why Full Spectrum Whole Plant (FSWP) extraction is more therapeutic. Some brands have much better percentages of different cannabinoids. Before we purchase our hemp we look at hundreds of labs to find the highest percentage of cannabinoids and terpenes, ensuring a finished FSWH rich in all natural cannabinoids and terpenes. CBD (Cannabidiol) is the second most prominent compound in cannabis and has finally taken center stage. Most people have heard of a cannabinoid called THC, which is the ingredient in cannabis that gets users high. Unlike THC, CBD (cannabidiol) is non psychoactive cannabinoid and does not cause a high. CBD has antipsychotic effects which means CBD works completely the opposite way of THC. Numerous studies suggest that CBD also acts to reduce the intoxicating effects of THC. About CBDa (Cannabidiolic Acid)The most common naturally occurring forms of CBD and THC are their acid forms, CBDa and THCa. Raw THCa is not psychoactive. It must be heated to form THC in order to become psychoactive. Raw cannabis is an historical component of the human diet. THC– Tetrahydrocannabinol- is the primary psychoactive component of the cannabis plant. This is the principle cannabinoid in marijuana that causes the psychoactive feeling of being “high”. CBD – Cannabidiol- is non-psychotropic. CBD is the most prevalent cannabinoid found in hemp. CBD has been used to treat epilepsy, and shown neuroprotective, antiepileptic, hypoxia-ischemia, anxiolytic, antipsychotic, analgesic, anti-inflammatory, anti-asthmatic, and antitumor properties. See more benefits of CBD here CBN– Cannabinol- is the primary product of THC degradation, and there is usually little of it in a fresh plant. CBN increases as THC degrades in storage and with exposure to light and air. Studies have suggested that CBN may be the most sedative of all of the cannabinoids representing a promising tool for the treatment of anxiety and stress related conditions. CBN may be effective at easing symptoms for patients with degenerative, motor neural diseases. CBG– Cannabigerol- is non-psychoactive. CBG is quickly converted to other cannabinoids through natural processes that occur within the cannabis plant, in general, most mature cannabis plants contain less than 1% CBG. CBC– Cannabichromene- is non-psychoactive and does not affect the psychoactivity of THC. CBC is the second most prevalent cannabinoid found in hemp. CBL– Cannabicyclol- CBL is known to occur as a degradation product of CBC. CBE– Cannabielsoin- CBE forms from CBD during the metabolic process, so it is a metabolite. |
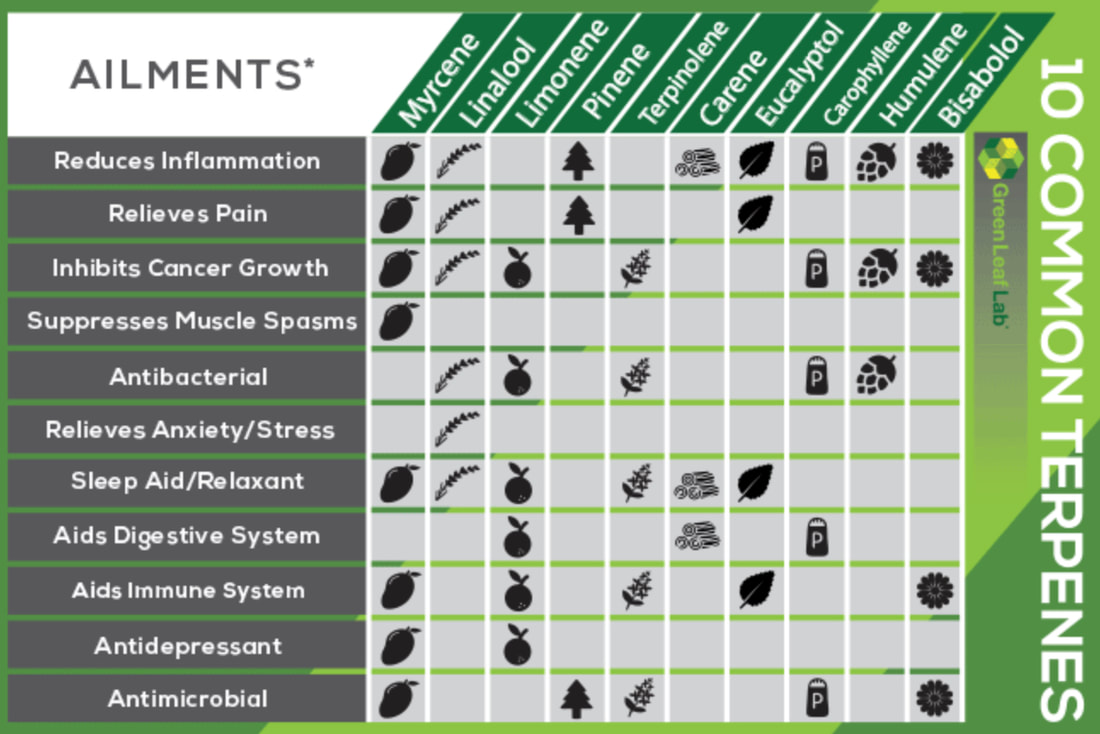
TERPENESTerpenes or isoprenoids, provide cannabis with its unique bouquet. The molecules are quite small and consist of repeating units of a compound called isoprene. Although less well-known than the major cannabinoids, terpenes play a vital role in the plant kingdom; they deter insect predation, protect plants from environmental stresses, and act as building blocks for more complex molecules, such as cannabinoids. Many terpenes act synergistically with other varieties of terpenes, and some either catalyze or inhibit formation of different compounds within a plant. Below are some of the major ones:
Different Types Of Terpenes Pinene– accounts for cannabis’ familiar odor, often associated with pine trees and turpentine. Pinene is the most common naturally occurring terpenoid and acts as both an anti-inflammatory and a bronchodilator. Linalool– has a floral scent reminiscent of spring flowers, but with spicy overtones. It possesses sedative properties and is an effective anxiety and stress reliever. It has also been used an analgesic and anti-epileptic. Limonene- is a dominant terpene in strains with a pronounced Sativa effect. It is also found in the rinds of citrus fruits. Limonene aids in the absorption of other terpenes through the skin and mucous membranes, and has been used to treat anxiety and depression. Myrcene– is the most prevalent terpene and is found in most varieties of cannabis. Myrcene concentration dictates whether a strain will have an Indica or Sativa effect. Strains containing over 0.5% of myrcene produce a more sedative high, while strains containing less than 0.5% myrcene have an energizing effect. Myrcene is also present in thyme, hops, lemongrass, and citrus, and is used in aromatherapy. Ocimene– is frequently used in perfumes for its pleasant odor. In nature, this terpene contributes to a plant’s defenses and possess antifungal properties. Terpinolene– has been shown to exhibit antioxidant and anticancer effects in rat brain cells. Studies with mice show that terpinolene has a sedative effect when inhaled. In addition, terpinolene is responsible for many of the floral notes found in Jack Herer varieties. Terpineol– is known for its pleasant smell and is often used in soaps and perfumes. It is known to have relaxing effects. Valencene– is present in Valencia oranges and contributes to cannabis’ citrus aroma. Caryophyllene– is the only terpene known to interact with the body’s endocannabinoid system (CB2). It produces anti-inflammatory and analgesic effects. Geraniol– Also present in geraniums, geraniol emits a rosey scent that makes it a popular perfume additive. It is an effective mosquito repellent and shows a potential protective effect against neuropathy. Humulene– contributes to the “hoppy” aroma of cannabis. This terpene acts as an appetite suppressant and exhibits potent anti-inflammatory activity. |
Bibliography
- Harvey, D. J. Journal of Ethnopharmacology,. J. Ethnopharmacol. 28, 117–128 (1990).
- Adams, R., Baker, B. R. & Wearn, R. B. Structure of Cannabinol. III. Synthesis of Cannabinol, 1-Hydroxy-3-n-amyl-6,6,9-trimethyl-6-dibenzopyran. JACS 62, 2204–2207 (1940).
- ElSohly, M. A. & Slade, D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 78, 539–548 (2005).
- Elsohly, M. A., Radwan, M. M., Gul, W., Chandra, S. & Galal, A. Phytocannabinoids. 103, (2017).
- Ahmed, S. A. et al. Cannabinoid Ester Constituents from High-Potency Cannabis sativa. J. Nat. Prod. 71, 536–542 (2008).
- Zulfiqar, F. et al. Cannabisol, a novel delta- 9-THC dimer possessing a unique methylene bridge, isolated from Cannabis sativa. Tetrahedron Lett. 53, 3560–3562 (2012).
- Radwan, M. M. et al. Isolation and Pharmacological Evaluation of Minor Cannabinoids from High-Potency Cannabis sativa. J. Nat. Prod. 78, 1271–1276 (2015).
- Ahmed, S. A. et al. Minor oxygenated cannabinoids from high potency Cannabis sativa L. Phytochemistry 117, 194–199 (2015).
- Pertwee, R. G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 153, 199–215 (2008).
- Izzo, A. A., Borrelli, F., Capasso, R., Di Marzo, V. & Mechoulam, R. Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb. Trends Pharmacol. Sci. 30, 515–527 (2009).
- Loewe, S. Marjiuana Activity of Cannabinol. Science (80-. ). 102, 615–616 (1945).
- Rhee, M.-H. et al. Cannabinol Derivatives : Binding to Cannabinoid Receptors and Inhibition of Adenylylcyclase. J . Med. Chem. 40, 3228–3233 (1997).
- Karniol, I. G., Shirakawa, I., Takahashi, R. N., Knobel, E. . & Musty, R. E. ·. Effects of delta-9-Tetrahydrocannabinol and Cannabinol in Man. Pharmacology 13, 502–512 (1975).
- Showalter, V. M., Compton, D. R., Martin, B. R. & Abood, M. E. Evaluation of binding in a transfected cell line expressing a peripheral cannabinoid receptor (CB2): identification of cannabinoid receptor subtype selective ligands. J. Pharmacol. Exp. Ther. 278, 989–999 (1996).
- Felder, C. C. et al. Comparison of the pharmacology and signal transduction of the human cannabinoid CB1 and CB2 receptors. Mol. Pharmacol. 48, 443–450 (1995).
- Pertwee, R. Pharmacology of cannabinoid receptor ligands. Curr Med Chem 6, 635–637 (1999).
- MacLennan, S. J., Reynen, P. H., Kwan, J. & Bonhaus, D. W. Evidence for inverse agonism of SR141716A at human recombinant cannabinoid CB1 and CB2 receptors. Br. J. Pharmacol. 124, 619–22 (1998).
- De Petrocellis, L. et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 163, 1479–1494 (2011).
- Wilkinson, J. D. & Williamson, E. M. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J.
- Dermatol. Sci. 45, 87–92 (2007).
- Siemens, A. J. & Turner, C. E. Marijuana research findings: 1980. NIDA Res. Monogr. Ser. 31 31, 167–198 (1980).
- Kargmanss, S., Prasitn, P. & Evans, J. F. Translocation of HL-60 Cell 5-Lipoxygenase. J. Biol. Chem. 266, 23745–23752 (1991).
- Appendino, G. et al. Antibacterial Cannabinoids from Cannabis sativa : A Structure - Activity Study. J. Nat. Prod. 71, 1427–1430 (2008).
- Qin, N. et al. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J. Neurosci. 28, 6231–6238 (2008).
- Scutt, A. & Williamson, E. M. Cannabinoids stimulate fibroblastic colony formation by bone marrow cells indirectly via CB2 receptors. Calcif. Tissue Int. 80, 50–59 (2007).
- Lee, S. Y., Oh, S. M. & Chung, K. H. Estrogenic effects of marijuana smoke condensate and cannabinoid compounds. Toxicol. Appl. Pharmacol. 214, 270–278 (2006).
- Osei-Hyiaman, D. Endocannabinoid system in cancer cachexia. Curr. Opin. Clin. Nutr. Metab. Care 10, 443–448 (2007).
- Weydt, P. et al. Cannabinol delays symptom onset in SOD1 (G93A) transgenic mice without affecting survival. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 6, 182–184 (2005).
- Zygmunt, P. M., Andersson, D. A. & Hogestatt, E. D. Delta 9-Tetrahydrocannabinol and Cannabinol Activate Capsaicin-Sensitive Sensory Nerves via a CB1 and CB2 Cannabinoid Receptor-Independent
- Mechanism. J. Neurosci. 22, 4720–4727 (2002).
- Jan, T. R., Farraj, A. K., Harkema, J. R. & Kaminski, N. E. Attenuation of the ovalbumin-induced allergic airway response by cannabinoid treatment in A/J mice. Toxicol. Appl. Pharmacol. 188, 24–35 (2003).
- Kalant, H. Smoked marijuana as medicine: not much future. Clin Pharmacol Ther. 83, 517–519 (2008).
- Gregg, J. M., Campbell, R. L., Levin, K. J., Ghia, J. & Elliott, R. A. Cardiovascular effects of cannabinol during oral surgery. Anesth. Analg. 55, 203–213 (1976).
- ELSOHLY, HARLAND, E., MURPHY, J. C., WIRTH, P. & WALLER, C. W. Cannabinoids in Glaucoma : A PrimaryScreening Procedure. Cournal Clin. Pharmacol. 21, 472S–478S (1981).
- Russo, E. B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 163, 1344–1364 (2011).